
Since each sample contains the same number of molecules (1.0mol of molecules), the sample of N2O5N2O5 has the largest mass. The compound with molecules containing the greatest number of atoms of N and O has the greatest molar mass. Which of the following additional information is most helpful in calculating the mole percent of XCl(s) and of ZCl(s) in the mixture? The molar masses of X and Z Which of the following best helps to explain why the atomic radius of K is greater than that of Br? The effective nuclear charge experienced by valence electrons is smaller for K than for Br A 1.0 mol sample of which of the following compounds has the greatest mass? The mass of a sample of any compound can be calculated by n×Mn×M, where n is the number of moles of molecules in the sample and MM is the molar mass (mass per mole) of the compound.
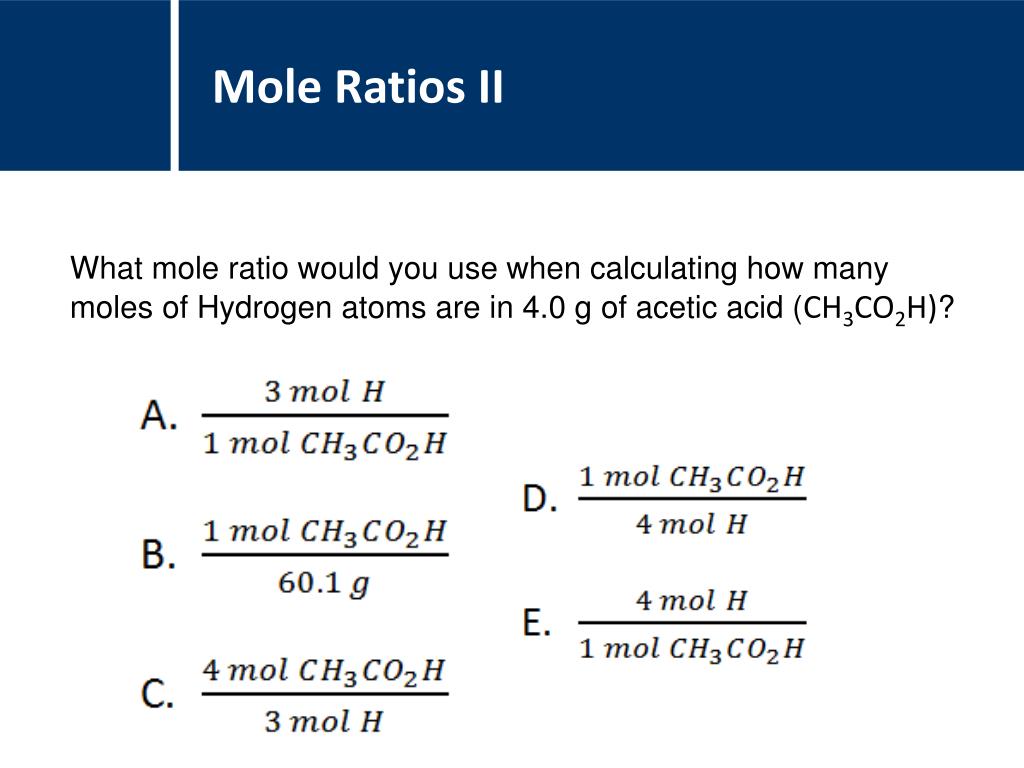
The percent by mass of X and the percent by mass of Z in the mixture is known. The molar mass of Cu A student obtains a mixture of the chlorides of two unknown metals, X and Z. Which of the following pieces of information is most useful for determining the number of Cu atoms in the sample? Assume that the pressure and temperature in the lab are 1.0atm and 25☌. Tip: You can also use mmol (millimoles) and mL (milliliter) In a lab a student is given a 21g sample of pure Cu metal. Which of the following questions about the compound can be answered using the results of the analysis? What is the empirical formula of the compound? Molarity moles of solute/solution (L) The results showed that the sample contained 36.0g of C and 6.0g of H.
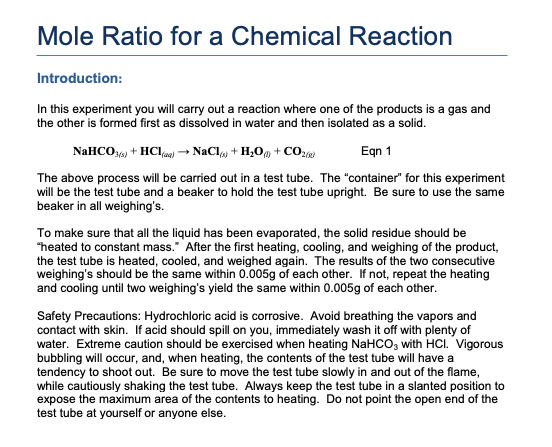
Which of the following scientific questions could best be answered based on the results of the experiment? Is the BaCl2(s)BaCl2(s) used in the experiment pure? A 42.0g sample of compound containing only C and H was analyzed. The student filters the BaSO4(s), rinses it, and dries it until its mass is constant. After completely dissolving the powder in 50.0mL of distilled water, the student adds excess Na2SO4(s), which causes a precipitate of BaSO4(s) to form, as represented by the equation above. Ba2+(aq)+SO42−(aq)→BaSO4(s)Ī student obtains a 10.0g sample of a white powder labeled as BaCl2. Which of the following best helps explain the trend of increasing atomic radius from N to Bi? The attractive force between the valence electrons and the nuclei of the atoms decreases. Which of the following questions can be answered from the results of the experiment? What is the molar mass of M? The atomic radii of the elements in the nitrogen group in the periodic table are given in the table above.

The student measures the mass of the compound that was formed. Then the student heats the sample in air, where it completely reacts to form the compound MO. Atomic mass spectrum shows relative abundance of each ion rough average to determine the element ie) image probable Mg or Al A student measures the mass of a sample of a metallic element, M.


 0 kommentar(er)
0 kommentar(er)
